
PEEK cranial implants are used so largely in cranioplasty due to their material properties in comparison with titanium.
Due to its properties like biocompatibility, strength, and flexibility, PEEK can be recommended for use in cranial implants because it provides advantages over metal in terms of weight and workability in surgery.
FDA Approval and Use of the VSP PEEK Cranial Implant is the First Additively Manufactured PEEK Implant to Receive FDA Approval for Cranioplasty to Repair Cranial Defects


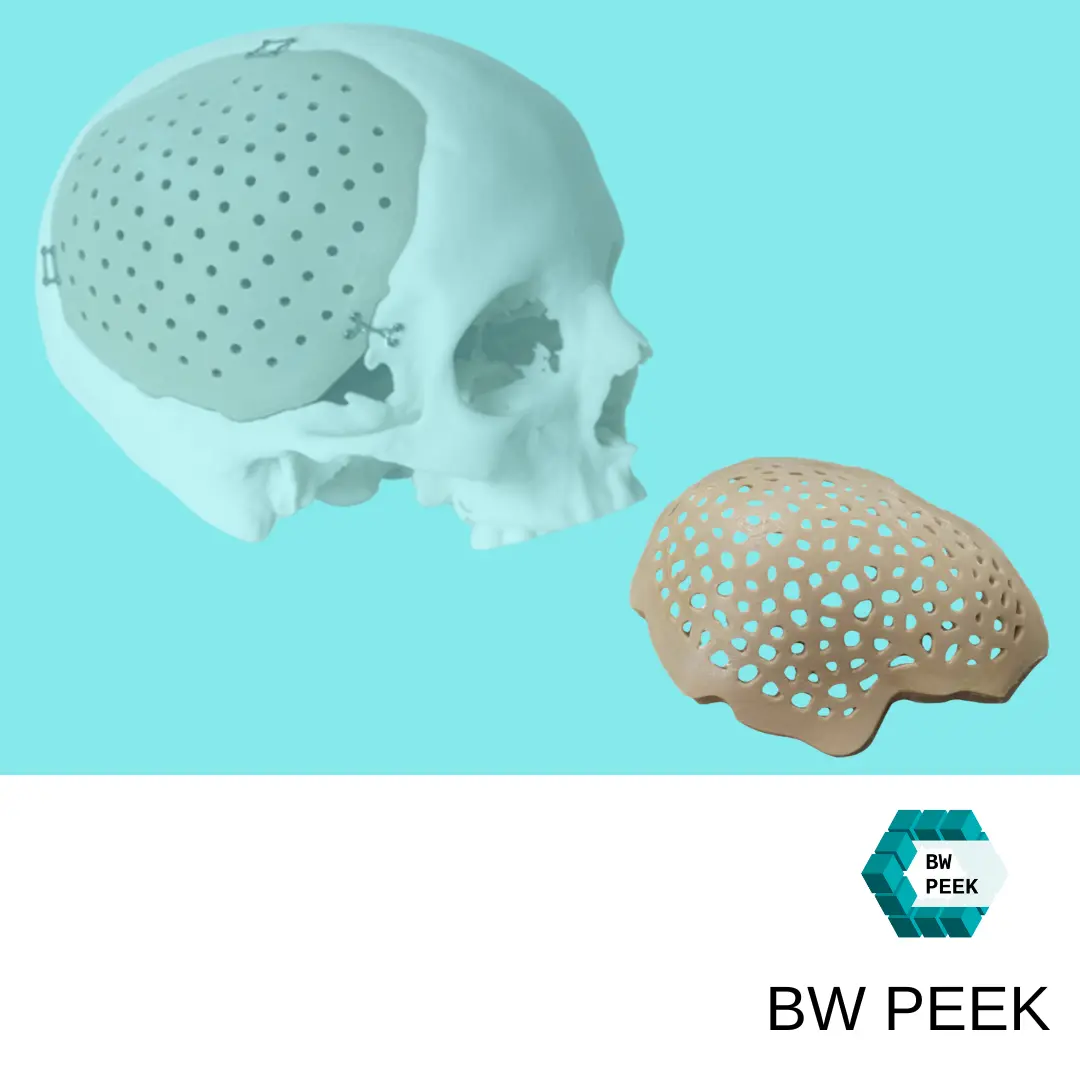

The U.S. Food and Drug Administration (FDA) has approved the VSP PEEK cranial implant solution, marking a significant milestone in the use of PEEK cranial implants. This is the first FDA-approved additively manufactured PEEK implant for use in cranioplasty to repair skull defects.
BW Medical mainly interested in applicative solution of medical polymer materials including PEEK in the medical field. The company has more than 18 years of experiences in engaging with the application of polymer materials like Peek and also research and development works on the materials and customers can avail various services from the company right from buying raw materials to ordering Original Equipment Manufactured goods.
BW Medical has more than 18 years from 2007 in advanced plastics, particularly about PEEK products in Medical industry so we can supply you with our professional advice and consideration.
In BW Medical, ‘SGS ISO13485’ quality management guarantee for the whole machinery exists and RoHS approval is available.