
The biocompatible polymer BW® PEEK is a medical implantable PEEK material that is certified with ASTM F2026 and Meets ISO 10993.
The material is made under quality management system in compliance with standards ISO 13485 and ISO 9001. BW® PEEK has applications in temporary and permanent implant applications such as – spinal fusion devices; anchor; cranial implants; dental implants; maxillofacial implants; various medical devices and parts.
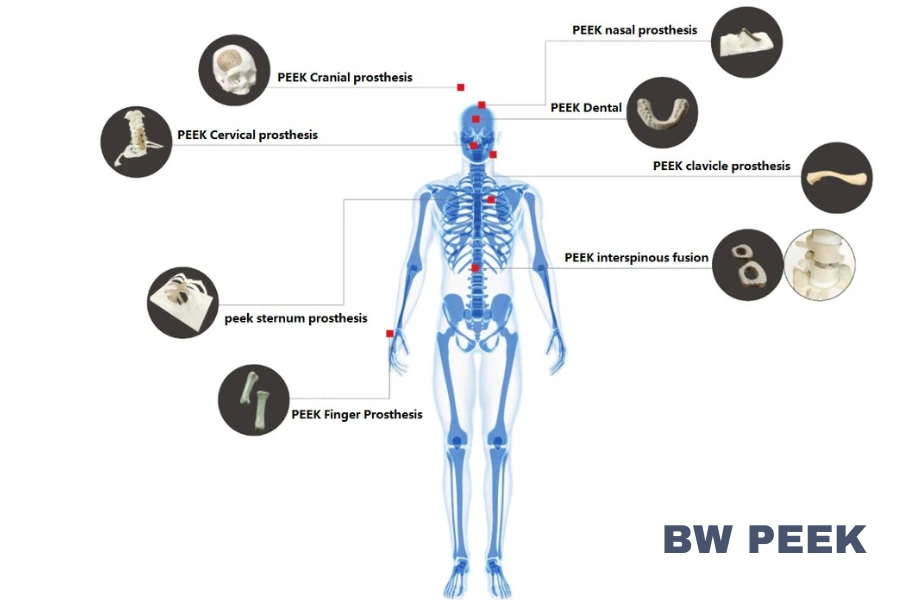

PEEK's high mechanical strength, elastic modulus close to that of human bone, and biocompatibility make it an ideal material for orthopedic implants.These implants provide good support and stability in the body.

PEEK medical materials can be used to manufacture PEEK hip, PEEK knee and PEEK prosthetic bones ,as peek medical have a modulus of elasticity close to that of human bone and are biocompatible, do not cause rejection, and can remain relatively stable in the body.
Referenced papers
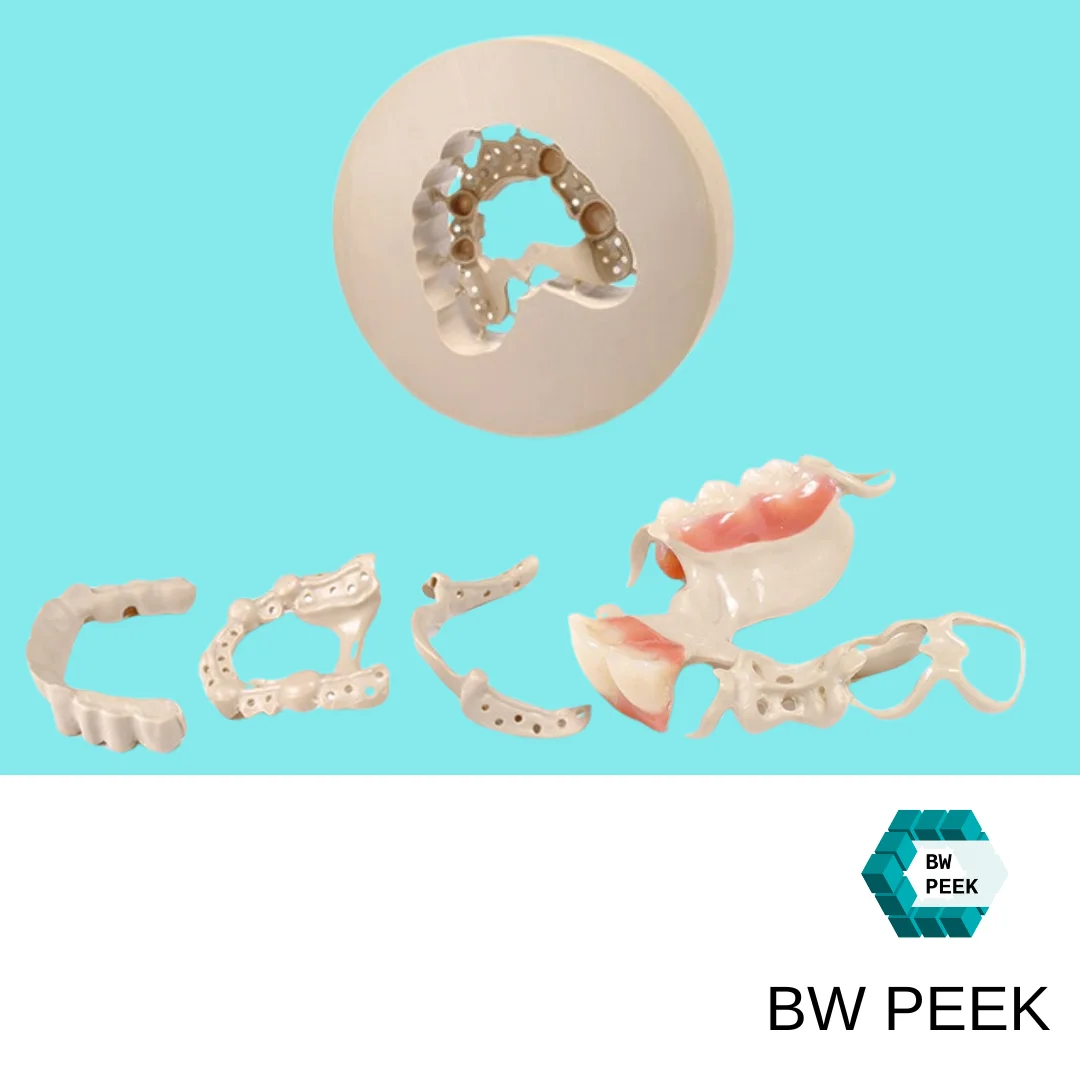
PEEK Medical blocks are the material of choice for dental restorations due to their aesthetics and biocompatibility, and are commonly used in the manufacture of the following dental products
PEEK has a modulus of elasticity very close to that of bone, avoiding the problem of stress masking that exists with metal implants.
PEEK has a melting point of 343°C and can be used in pressurized hot water or steam at 300°C. PEEK can be autoclaved up to 3,000 cycles at 134°C, a characteristic that makes it useful for the production of surgical and dental devices that require high levels of sterilization and repeated use.
PEEK is highly resistant to most chemicals and is not easily corroded.
PEEK does not cause adverse reactions in the human body and is suitable for use as an implant material.
PEEK has good penetrability to X-rays and does not produce metal artifacts, making it easy for post-operative observation.